Molecular Surface Properties of Polymers
Personnel: Miguel A. Amat
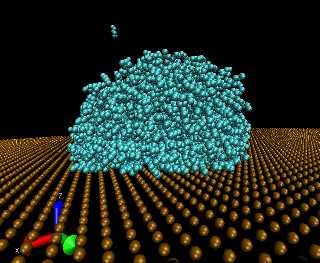
The simple action of placing a liquid droplet on a solid surface may result in a variety of intriguing and complex interfacial phenomena. It is well known that liquids on surfaces form a contact angle, &thetac. Depending on the properties of the materials used, the surface may be classified as wetting; when the angle formed by the contacting liquid takes on values within the range, 0° &le &thetac < 90°, or it may be classified as non-wetting; when the contact-angle takes values that are &thetac &ge 90°. In addition, special types of surfaces exist which exhibit apparent contact-angle values in excess of 150°. These surfaces are classified as superhydrophobic; when the contacting liquid is water, or as superoleophobic; when the contacting liquid is an organic hydrocarbon. Surfaces that contain morphological irregularities such as roughness and other heterogeneities may also exhibit what is known as contact-angle hysteresis — a difference between the advancing, &thetaa, and receding contact angles, &thetar. While contact-angle phenomena are manifested and observed at macroscopic length scales, their origin clearly stems from interactions taking place at the molecular level. Despite their ubiquitous presence in nature and in industrial applications, a complete understanding of contact-angle phenomena and the factors that govern them is not satisfactory at the present time.
A major goal in this project is to develop new simulation models aimed at providing a basic understanding of the factors that control contact-angle phenomena in liquids interacting with solid surfaces. This is achieved through the development of computational tools that probe the behavior of polymeric surfaces and their interaction with liquids. Efforts are made to provide simple yet meaningful model representations that highlight the properties of interest without sacrificing the essential aspects of the underlying physics.