Electrospun Fiber Scaffolds for Medical Use
Personnel: Joseph L. Lowery, Neta Datta
Tunable fiber diameters, large porosity and specific surface area, and the ability to generate fibers from a wide range of materials make electrospinning an excellent candidate for producing artificial extracellular matrix (ECM) analogues for tissue engineering. The artificial ECM acts as a template for the replication of cells and collagen production, leading the way for regeneration of significantly damaged or non-regenerative tissues. Designing materials with modifiable pore diameters and void fractions, as well as controllable mechanical properties, is an important step towards achieving tissue repair; these architectural features are expected to affect signaling, differentiation, and replication of cells implanted into the artificial ECM. For tissues that are capable of some level of regeneration, electrospun materials can be constructed in a way to mechanically assist the regenerative process by limiting the need for medical suturing and providing an unimpeded pathway for regeneration to occur.
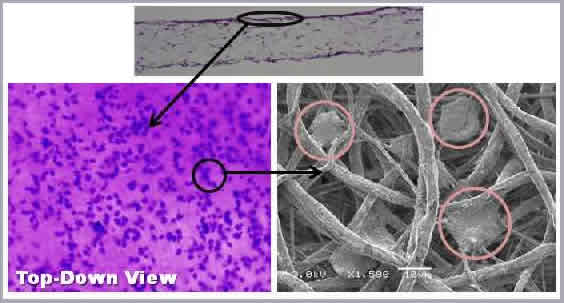
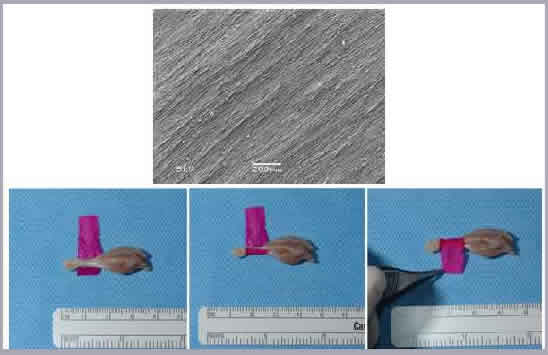
Using a perfusion bioreactor, we have successfully seeded both juvenile bovine chondrocytes (JBC) and human dermal fibroblasts (HDF) into 400-600 μm-thick tissue scaffolds. Four week studies of JBC reveal migration and proliferation of the cells throughout the thickness of the scaffold, as well as significant ECM deposition. HDF studies have found that perfusion seeding induces greater cell retention and proliferation over traditional droplet-seeding techniques. In addition, an increase in pore diameter was found to significantly impact cell growth. We have also successfully created hollow nerve guidance pathways composed of a blend of electrospun poly(ε-caprolactone) (PCL) and poly(lactide-co-glycolide) (PLGA) to aid in the regeneration of both sciatic and spinal cord tissue [Panseri et. al; BMC Biotechnology 2008]. Finally, core-shell electrospinning of aligned poly(ethylene oxide) (PEO) / silk fibroin nanofibers and subsequent PEO removal can be used to generate a surgical wrap to aid in the repair of damaged tendons and ligaments. Crosslinking with a photoactive dye bonds the electrospun template to the tissue. This results in reduced adhesion formation and improves mechanical properties of the repair, reducing the need for extensive suture repair.